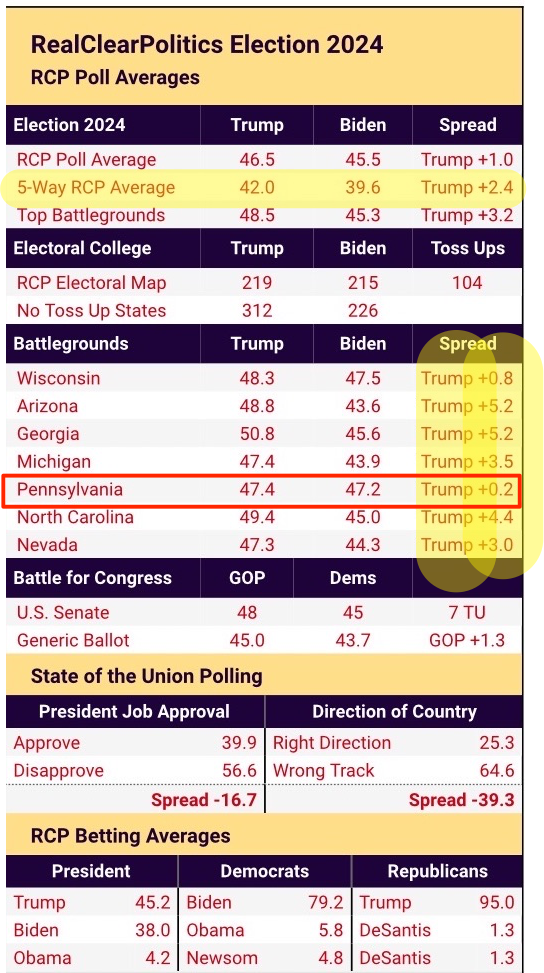
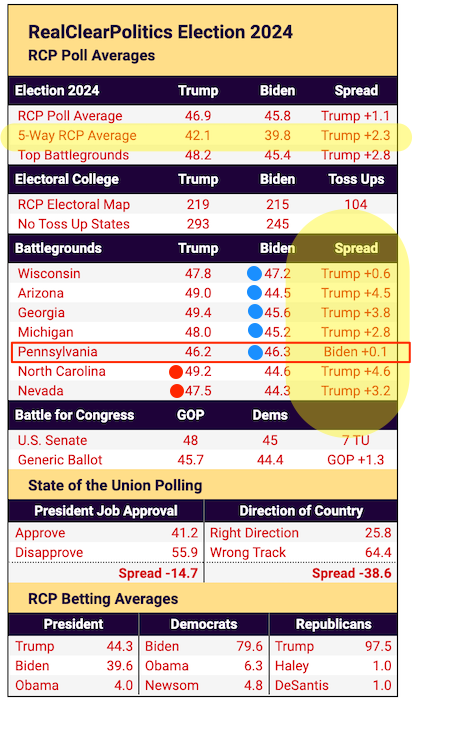
By Leroy Leo and Michael Erman
(Reuters) -The U.S. Meals and Drug Administration on Tuesday authorised Merck’s remedy for adults with hypertension on account of constriction of lung arteries, including one other potential blockbuster drug to the pharmaceutical large’s portfolio.
Shares of Merck have been up greater than 5% in prolonged buying and selling.
The remedy, branded Winrevair, is authorised for treating pulmonary arterial hypertension (PAH), which impacts about 40,000 individuals in america.
“We look ahead to making a big distinction for these sufferers which can be left with a illness the place the 5 yr mortality is 43%,” Jannie Oosthuizen, president of Merck’s U.S. Human Well being enterprise, instructed Reuters.
Winrevair will carry a listing worth of $14,000 per vial, Oosthuizen stated. In accordance with information from the corporate’s trial, most sufferers will use a single vial each three weeks, which might translate to $238,000 per yr.
The drug maker expects to have the ability to deliver the drug to the market by the tip of April.
Merck acquired the rights to Winrevair as a part of its $11.5 billion acquisition of Acceleron Pharma (NASDAQ:) in 2021. It has been beefing up its portfolio of cardiovascular medicine as a part of its technique to counter a attainable hit to gross sales to its most cancers remedy Keytruda, the world’s prime promoting medication, from biosimilars later within the decade.
Winrevair turns into the primary remedy to safe FDA approval from its class of medicine, which goal a sort of protein known as activin that result in larger ranges of a follicle-stimulating hormone related to the illness.
It’s the second drug to be authorised for PAH in lower than every week, after Johnson & Johnson (NYSE:)’s Opsynvi – a mixture of J&J’s older PAH drug Opsumit and generic drug tadalafil – acquired the FDA’s nod late Friday.
PAH is attributable to a constriction of arteries within the lungs, resulting in hypertension and signs comparable to shortness of breath, chest ache and dizziness.
The hypertension additionally makes the guts work more durable to pump blood, ultimately inflicting coronary heart failure.
In October, J.P. Morgan analyst Chris Schott (ETR:) had estimated the remedy would attain peak gross sales of $3 billion to $4 billion.
Approval for Merck’s drug was primarily based on a 24-week lengthy late-stage trial of 323 sufferers with PAH.
Within the trial, sufferers handled with the drug confirmed a big enchancment in train capability, rising their 6 minutes strolling distance by 40.8 meters, in comparison with the placebo.